Effective Strategy Reducing Accidental Needlestick Injuries IONM Procedures Case Study 2019
Table of Contents
An Effective Strategy for Reducing Accidental Needlestick Injuries During Intraoperative Neuro Monitoring Procedures: Case Study
Grace Padilla-Kastenberg, MPH, Brent Tyler, MD, CNIM
ABSTRACT
Injuries incurred by accidental needlesticks continue to pose a serious problem in healthcare settings and particularly during surgical procedures. Intraoperative Neuro Monitoring (IOM) is a unique surgical practice that employs non-cannulated (subdermal) needles to monitor nerve function during surgical procedures. This paper evaluates data reported from a single IOM surgical practitioner demonstrating the incidence and prevalence of accidental needlestick injuries (NSI) during IOM procedures over a thirteen-year period. The data collected compares the efficacy of various medical adhesives that are used to affix needles to patients as they pertain to reducing needlesticks during IOM procedures. Due to ongoing needlesticks experienced by the clinical practice, a new adhesive solution was introduced. A significant (90%) reduction in needlesticks were experienced by the clinical practice as compared to previous needle fixation methods. This paper demonstrates how risk management strategies can positively impact outcomes as they pertain to accidental needlestick injuries during IOM procedures.
Keywords: Sharps Injury, Needlesticks, Safety Alert, Bloodborne Pathogens, Subdermal Needles, Needle Count, IOM, IONM, Neuromonitoring, Operating Room, Surgical Settings.
INTRODUCTION
Sharps injuries are responsible for placing healthcare workers (HCW) at risk through the transmission of bloodborne pathogens like Hepatitis, HIV and other infectious diseases. 1 An estimated 380,000 instances of sharps and needlestick injuries take place each year in hospital settings. 2 A large portion of these injuries (48%) take place in the operating room (OR), and are incurred by physicians, nurses, surgical technicians and other surgical personnel. 3 This also includes anesthesiologists and CRNA’s. Nearly 30% of injured HCW are incidentally affected by sharps and needlestick injury due to their exposure in the operating room.
While non-cannulated subdermal needles typically have a lower probability of transmitting bloodborne pathogens, non-cannulated devices in total continue to account for nearly sixty percent (60%) of sharps and NSI in all hospital settings. Of these injuries, thirty-three percent (33%) are caused by non-cannulated needles. These statistics are particularly important for personnel working in IOM where many noncannulated subdermal needles are placed on the body during surgical procedures. IOM personnel and other OR HCW remain at risk to the transmission of bloodborne pathogens from these devices. In the event that IOM HCW are accidentally stuck by a subdermal needle, these individuals require the same recommended prophylactic treatment protocol that other HCW would receive. They also experience the same psychological effects that someone who is stuck with a syringe or cannulated needle might experience. 4
In the few published studies on IOM and NSI, IOM clinicians report that they have experienced needlesticks at least once by subdermal needles during the course of their career. If they have not, they have almost universally reported that others in the OR have experienced these injuries. This anecdotal evidence is supported by several studies addressing this unique HCW hazard. 5 , 6 The IOM community has acknowledged an under-reporting of these injuries, much like is found in other healthcare sectors where needles and other sharps are employed that are at-times causal in accidental needlesticks.
The development of this white paper was spearheaded by Beacon Monitoring, a neuromonitoring clinical practice located in the State of Alaska. The IOM clinician pursued a solution to address the occurrence of NSI during the course of his practice. The procedural and needlestick data gleaned from this practice, serves as the basis of this paper and subsequent analyses.
OBJECTIVES
Analyze a data set spanning thirteen (13) years from an individual IOM clinical practice on the topic of accidental NSI in surgical settings and address changes over time with the use of a new strategy. The results and analyses of the data may provide a better understanding of the challenges associated with needlesticks and present a safety solution which can be shared with IOM Technicians/Clinicians, OR Managers, Hospital Risk Managers and Clinical OR Nurse Educators. METHODS An analysis was conducted with self-reported needlestick incidence data collected over a thirteen (13) year period of time within an individual surgical IOM practice. During this span, a variety of adhesive solutions were employed to affix subdermal needles to patients. A combination of paper tape, silk tape and poly tape (Micropore™, Transpore™, and other needle fixation adhesives) were employed from 2006 through the close of 2012. During each surgical procedure, a single piece of one of the above adhesive options was affixed to either a single subdermal needle or a pair of subdermal needles. Over the thirteen year period of time a variety of subdermal needles were used including single and twisted pair subdermal needles from the following manufacturers: Rhythmlink® , Ambu® , Medtronic® , Neurovision Medical Products® and Friendship Medical.
The needle fixation and removal protocol included the following: (1) pre-planning and conveyance to OR personnel that needles were being applied to the patient at anatomical sites; (2) applying the needle(s) at a specific anatomic site; (3) creating a strain relief loop near the needle hub; (4) applying the selected adhesive with minimal handling, ensuring security of the adhesive; (5) alerting HCW in the OR that needles were present; (5) chronicling the number of needles placed in the patient at the close of the case, referencing the needle count with each removal; (6) removing the needles by lifting them by the wires with one hand applying counter pressure with the other; (7) disposing of the needles in a sharps disposal container; and (8) thereafter confirming to the assigned circulating nurse that all needles were removed.
From January 2013 through December 2018 an alternative adhesive fixation solution that included a safety identifier, was introduced (NeedleTape® ). The process of needle fixation and removal followed the same protocol as described and listed above. This alternative was selected by the IOM clinical practice due to the incorporation of a safety identifier with the purpose of enhancing visual awareness to the presence of needles.
A variety of metrics were analyzed and compared between 2006 through 2012, and 2013 through 2018. This included the total number of needlesticks that occurred over each time period and a weighted average of the total subdermal needles used within each time period.
RESULTS
Thirteen-year Data Collection-Beacon Monitoring
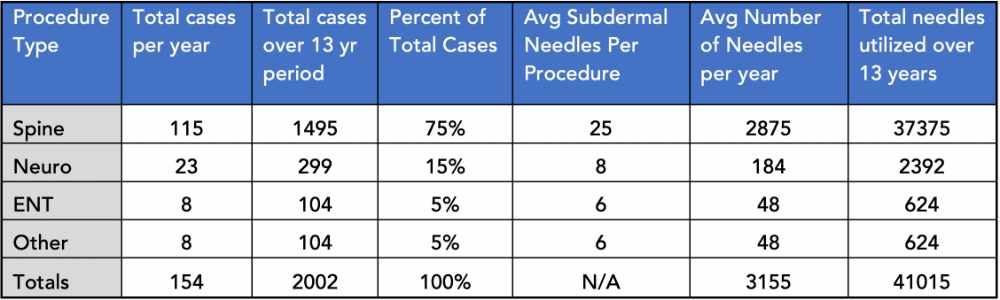
A total of 2,002 IOM procedures took place during the thirteen (13) year span (approx. 154 per year) employing multiple adhesive fixation options, with a total weighted average of twenty (20) subdermal needles used per procedure (avg needles per year x avg procedure per year). The estimated total number of subdermal needles during this period of time was 41,015.
Needlesticks (NSI) by Fixation Type
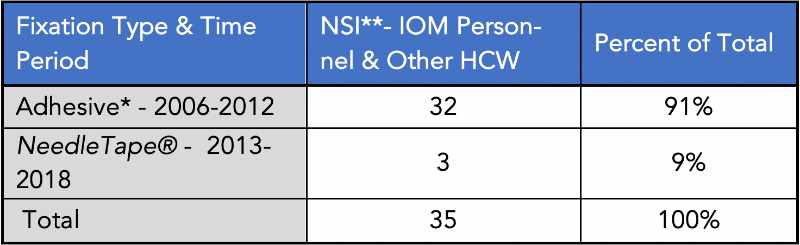
*Micropore™, Transpore™, 3M silk tape **Needlestick Injury
A total of thirty-five (35) subdermal needlesticks were reported during the span of thirteen years (2006- 2018). Thirty-two (32) needlesticks were reported during the early period from 2006-2012. During the period where NeedleTape® was employed, (2013-2018), three (3) subdermal needlesticks were reported. Thus, the IOM clinical practice experienced close to a 90% reduction in NSI with the use of NeedleTape®. as compared to the other fixation types over a similar period of time.
Additional NSI data included a breakdown of NSI reported by the IOM clinician and NSI incurred by other HCW during IOM procedures. As stated above, a total of thirty-five (35) NSI were reported during the 13- year span. Eleven (11) of these subdermal NSI were incurred by the IOM clinicians; the remaining twentyfour (24) were incurred by other HCW during the course of surgery.
CONCLUSION
Engineering controls are consistently recommended as a strategy to reduce unintended needlesticks.7 The CDC estimates that between 62% to 88% of sharps injuries can be prevented simply by using safer medical devices 8 . Since adopting the NeedleTape® product, this IOM practice and involved HCWs experienced a significant decrease in the incidence of overall subdermal needlesticks. This reduction in needlestick injuries could be attributed to a higher level of awareness due to the integration of the NeedleTape® product, in addition to the inherent benefits afforded by the product itself. The clinical IOM practice also reported a significant reduction in the incidence of inadvertent needlesticks to other HCW’s within the operating room settings where IOM services were provided since integration of the safety product into the practice.
After integrating NeedleTape® , the clinical IOM practice reported an enhanced relationship between the surgical staff where increased awareness is a benefit to all in the surgical suite. The practice also reported a reduction in liability due to their demonstration of a safety commitment to the health and welfare of HCW in the OR.
As reported by the clinical IOM practice the following are reasons why the NeedleTape® , product helps to address the issue of needlesticks within IOM; (1) provided favorable adhesive fixation that held needles in place throughout the entire procedure; (2) assisted in charting locations for post close needle counts; (3) provided ease of overall use for the IOM clinician; (4) was cost effective and particularly when considering the cost of treating a needlestick; (5) allowed for visualization of IOM subdermal needles for the IOM clinician due to product transparency while also warning all HCW that needles are present; (6) enhanced relationships with anesthesia, nurses and other operating room based HCW by providing an alert and demonstrating that the IOM clinician is looking out for their safety.
There were limitations to this study including self-reported data; a singular clinical practice; and slightly unequal time frames used for comparison. Further studies are needed to better understand the scope of the NSI problem within IOM and other safety solutions.
Incidence of sharps injuries in surgical units, a meta-analysis and meta-regression. J osVerbeek M D,PhD, Prativa Basnet MPH,BPH Cochrane Work Review Group, Finnish Institute of Occupational Health, Kuopio, Finland. 2 Workbook for Designing, Implementing & Evaluating a Sharps Injury Prevention Program. Accessed 4/18/18 https://www.cdc.gov/sharpssafety/pdf/sharpsworkbook_2008.pdf 3 International Safety Center. U.S. EPINet Sharps Injury and Blood and Body Fluid Exposure Surveillance Research Group. Sharps Injury Data Report for 2017; 31 hospitals contributing data, 1339 total injuries. Report available at https://internationalsafetycenter.org/wp-content/uploads/2018/10/Official-2017-NeedleSummary.pdf.
Needlestick injuries in the United States. Epidemiologic, economic, and quality of life issues. Lee JM, Botteman MF, Xanthakos N, Nicklasson L. AAOHN J. 2005 Mar;53(3):117-33. 5 Intraoperative Neurophysiological Monitoring Poses Needlestick Injury Risk to OR Personnel and Patient. Abramowitz, E., Spinelli, Wecksell, Nair, Catalano. Am J. Infect Control 2008;36(10):753-6. 6 Risk of needle-stick injuries associated with the use of subdermal needle electrodes during intraoperative neurophysiologic monitoring. Tamkus, A.,Rice, K. J of Neurosurgical Anesth: Jan 2014-Vol 26-Issue 1-p 65-68.
OSHA Healthcare Wide Hazards-Needlestick/Sharps Injuries. https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=STANDARDS&p_id=10051 Accessed 11/15/18. 8 Bloodborne Pathogens and Needlestick Prevention. Safety and Health Topics. Available at: https://www.osha.gov/sltc/bloodbornepathogens/evaluation.html. Accessed 10/18/18.
Disclosures:
About Beacon Monitoring
Beacon Monitoring lead clinician Brent Tyler maintains an active membership in the ASNM (American Society of Neuro Monitoring). Additional work includes conducting a comparative study of various IOM modalities in Carotid surgery, and providing non-IOM neurodiagnostic services-exams in clinical settings. Contact: Brent Tyler, CNIM, President Beacon Neuromonitoring, admin@beaconmonitoring.net
About the Author
Grace Padilla-Kastenberg is a public health advocate with experience in the development of occupational health and public health promotion prevention strategies. Currently serves as President of Marea Enterprises who is active in developing strategies to assist in the reduction of needlesticks. NeedleTape® its signature technology has been employed in various health care settings since 2012 including within IOM.
Contact: Grace Padilla-Kastenberg, MPH, President Marea Enterprises, grace@needletape.com
Glossary and Definitions: Bloodborne Pathogens: Pathogenic microorganisms that are present in human blood and can cause disease in humans. These pathogens include, but are not limited to, hepatitis B virus (HBV) and human immunodeficiency virus (HIV).Cannulated: In this case, a needle configuration with a hollow bore HCW: Health Care Worker (Generally directly involved in patient care or contact) IOM: Intraoperative Monitoring (AKA IONM – Intraoperative Neuromonitoring) Needlestick: An accidental event whereby a needle inadvertently pierces the skin of a person (generally non-patient) NSI: Needlestick Injury Subdermal Needles: Needles designed for placement into the sub cutaneous layers or muscles of patients for use in patient monitoring Universal Precautions: An approach to infection control to treat all human blood and certain human body fluids as if they were known to be infectious for HIV, HBV and other bloodborne pathogens, (Bloodborne Pathogens Standard 29 CFR 1910.1030(b) definitions)
DOWNLOAD PDF – https://mareaenterprises.com/wp-content/uploads/2022/08/Reducing_Accidental_NS_IOM_Padilla-Kastenberg-MareaEnterprises_6.11.19_F.pdf


